| The Problem: Part 1
The early embryos of the camelids mature at a faster rate than those of other
livestock species, and they break
out of the zona pellucida, a process called hatching, before they reach the
uterus. That means the early embryos
obtained non-surgically from camelids are much larger and contain much more
water than the embryos flushed
from cows. These early camelid embryos are basically a thin spherical
envelope of living cells surrounding a
central volume of aqueous solution. That makes them very difficult
to freeze using conventional methods that
depend on osmotic penetration of the envelope by cryoprotectant molecules.
Camelid Embryo Cross-Section
The entire central volume of this sphere is water solution.
All the cryoprotectant must pass through the envelope. |
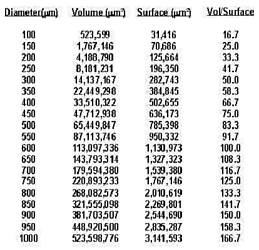
Ratio of Volume to Surface of a Sphere
Bovine Emb Dia.=~100 microns
Camelid Emb Dia.=500-1000 microns |
Cryoprotectants (glycerol, ethylene glycol, etc.) are toxic to living cells,
so the time of exposure to high concen-
trations of cryoprotectant agents (CPAs) must be limited. Because movement
of CPAs across a membrane
such as the envelope of a camelid embryo proceeds at a given rate per unit
of surface, exposure time increases
dramatically as the diameter of the embryo increases. In a nutshell,
it takes so long to raise the concentration of
CPA in the central volume high enough to prevent ice crystal formation
that the living cells are killed by the
cryoprotectant. It is the ratio of volume to surface of these large
embryos that is the basic problem in freezing
them.
After thousands of hours of freezing experiments with the hatched blastocysts
of llamas, it became obvious to me
that it would be necessary to take control of the central volume in order
to freeze these embryos successfully.
That meant finding a way to penetrate the envelope in order to inject CPA
and remove the aqueous solution
inside.
The Problem: Part 2
The envelope of living cells in these late-stage hatched blastocysts of the
camelids is amazingly difficult to
penetrate. It is stretchy and flimsy, but very tough. Trying
to hold the embryo from one side while you inject
from the opposite side (as you can do with an oocyte or an embryo still in
the zona) just doesn't work. Imagine
trying to push the tip of a ballpoint pen through the material of a nylon
stocking and you'll have a rough idea of
the difficulty involved in penetrating this envelope of cells with a
micropipette.
The Solution
After many trials, a coaxial design, with the injection pipette presented
from inside the holding pipette, worked
best. This combination pipette was soon known as the Dracula pipette,
because of the suck-and-puncture func-
tion it provided. It took almost a year to reach the current design,
the Dracula 1000, which makes routine
injection into the inside of the envelope and aspiration of the liquid inside
the envelope possible.
It was only the third embryo I tried to freeze using the Dracula that resulted
in the first live baby. That was Frio,
the first frozen/thawed baby in history in the South American camelids.
Frio, a male, was followed by Helada, a
female baby from a frozen embryo.

Frio, a male, on the left. Helada, a female, on the right.
The basic idea in freezing these embryos is to inject cryoprotectant directly
into the central volume, then remove as
much of the resulting solution as possible. This allows for fairly
rapid osmotic adjustment of the concentration of
cryoprotectant inside the envelope and inside the living cells with the movement
of only small volumes of water and
CPA through these membranes. That means the cells are exposed to CPA
for a shorter time and the intra-cellular
concentration of CPA can be raised higher while total pre-freeze exposure
(time x concentration) of the cells to
CPA is still significantly reduced.
As a major portion of the exposure time of these cells to CPA ordinarily
occurs after the thaw, we inject a diluent
solution into the envelope to re-inflate it, again using the Dracula, immediately
after rapid warming. Because there
was only a small volume of solution inside the envelope during the freezing
process, and only a very small amount of
cryoprotectant, re-inflation with culture medium results in instantaneous
dilution of the internal CPA below toxic
levels. The remaining CPA molecules can migrate out through the envelope
harmlessly over an extended period of
time, even after the thawed embryo is transferred into the uterus of a surrogate
dam.

This poster was presented at the January, 2006 meeting of the International
Embryo Transfer Society (IETS)

The Dracula system set up and ready for action.

A closer look. The micromanipulator is only used for final positioning
of the Dracula.
The advance and retraction of the injection pipette is done by hand.
|




